Saponification and Cleansing Agents: Detailed Guide
Understanding Saponification: The Chemistry of Soap-Making
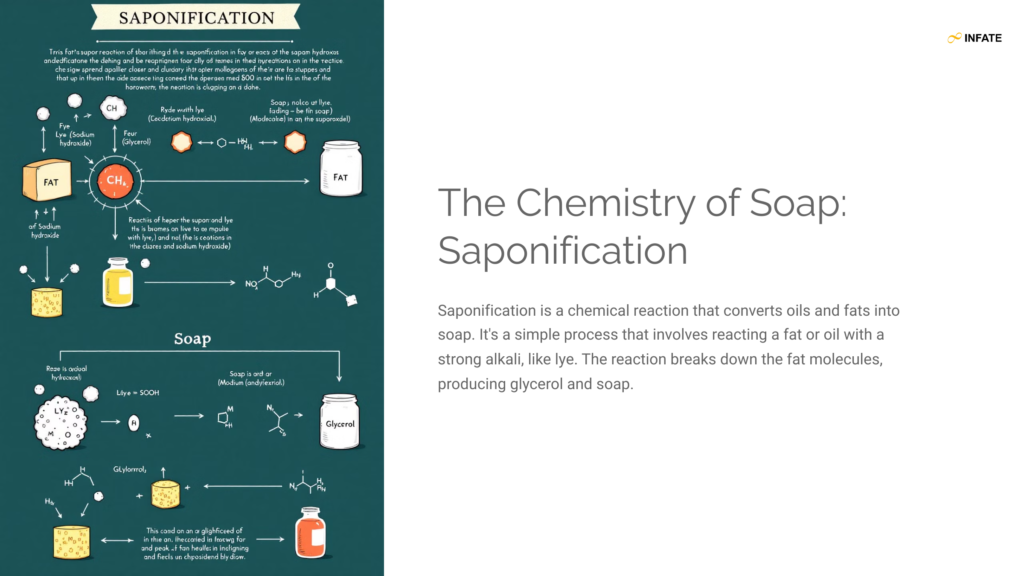
Saponification is a vital chemical process where fats or oils (esters of fatty acids) react with a strong base like sodium hydroxide (NaOH) to create soap and glycerol. This reaction forms the backbone of traditional soap-making.
General Equation for Saponification:
Fat/Oil (Ester) + Base (NaOH) → Soap (Salt of Fatty Acid) + Glycerol
Example of Saponification Reaction:
C3H5(COOR)3 + 3NaOH → 3RCOONa + C3H5(OH)3
- Triglyceride (fat/oil): C3H5(COOR)3
- Soap (sodium salt of fatty acid): RCOONa
- Glycerol: C3H5(OH)3
Soap vs. Detergent: Key Differences You Should Know
| Property | Soap | Detergent |
|---|---|---|
| Definition | Sodium or potassium salts of fatty acids. | Synthetic cleaning agents derived from petroleum. |
| Source | Made from natural fats and oils. | Made from petrochemicals or synthetic sources. |
| Chemical Structure | Contains a carboxylate group (-COONa). | Contains a sulfonate (-SO₃Na) or sulfate (-OSO₃Na) group. |
| Water Hardness | Ineffective in hard water; forms scum. | Effective in both soft and hard water; no scum formation. |
| Environmental Impact | Biodegradable. | Can be non-biodegradable and harm aquatic life. |
| Cost | Generally cheaper. | Usually more expensive. |
How Do Soaps and Detergents Clean?
Cleansing Action of Soap:
- Structure: Soap molecules consist of a hydrophobic tail (water-repelling, grease-attracting) and a hydrophilic head (water-attracting).
- Process: Soap forms micelles in water, trapping grease and dirt. These micelles are rinsed away during washing.
- Limitation: Soap reacts with calcium and magnesium ions in hard water, forming scum.
Cleansing Action of Detergents:
- Structure: Detergents use sulfonate or sulfate groups instead of carboxylate groups.
- Process: Detergents form micelles, trapping grease and dirt. They remain effective in hard water and do not form scum.
- Advantage: Suitable for both soft and hard water conditions.
Key Insights: Soaps vs. Detergents
Both soaps and detergents remove dirt through micelle formation, but their differences make each suitable for specific applications:
- Soaps: Eco-friendly and biodegradable, ideal for sustainability-conscious users.
- Detergents: Perform better in hard water and offer greater versatility, though they may pose environmental risks.
Choose based on your priorities—environmental impact or cleaning efficiency.


