Essential Water Properties – A Must-Know Guide for Grade 9 Chemistry
By rajeevvkalra | Category: Science
Water plays a crucial role in sustaining life and shaping our planet. Its ability to exist in different forms and its unique thermal and chemical properties make it an important topic for students. In this comprehensive article, we will cover important water-related concepts aligned with the Grade 9 Chemistry curriculum.
1. Water’s Physical States Explained
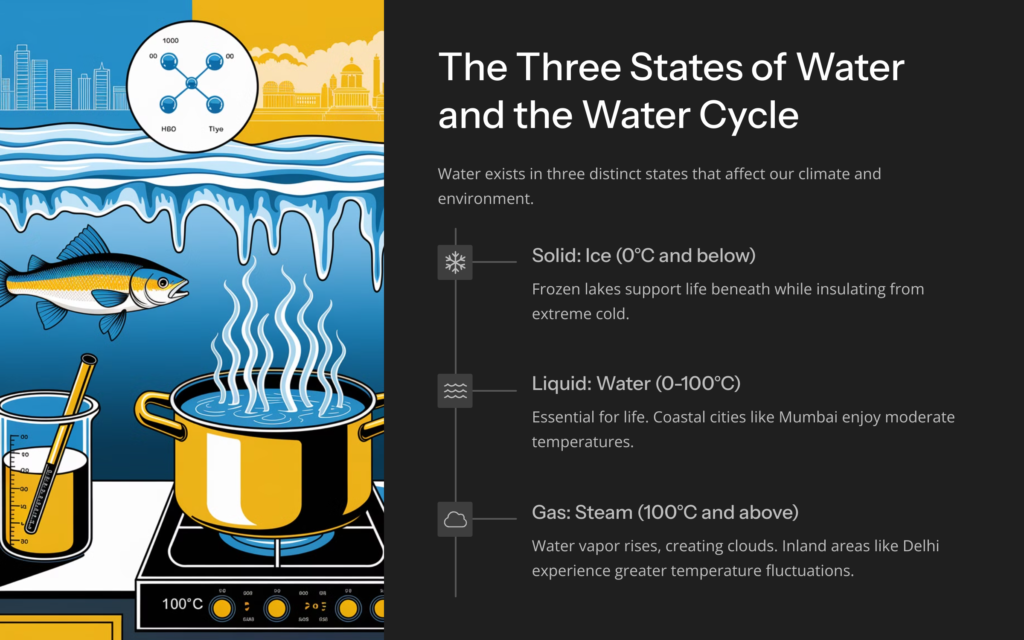
Water can exist in three physical forms:
- Ice (Solid): Below 0°C, water transforms into ice, forming glaciers and icebergs.
- Liquid: Between 0°C and 100°C, it remains a liquid, essential for rivers, oceans, and living organisms.
- Steam (Gas): Beyond 100°C, water vaporizes into steam, playing a key role in the water cycle.
2. Why Water is a Compound
This molecule is made of two hydrogen atoms and one oxygen atom (H2O), chemically bonded in a fixed ratio, making it a stable compound.
3. Influence of Water on City Temperatures
(a) Why Mumbai and Chennai have milder winters than Delhi
Coastal cities like Mumbai and Chennai experience stable temperatures due to the moderating influence of nearby oceans, unlike Delhi which is inland and more prone to extremes.
(b) How Water Regulates Body Temperature
Due to its ability to absorb significant amounts of heat without large temperature changes, water helps in maintaining internal body temperature.
4. Universal Solvent Nature
Because of its polarity and ability to form hydrogen bonds, water can dissolve a wide variety of substances, earning it the title of a universal solvent. Learn more.
5. What Triggers Torrential Rain Hazards
Heavy rainfall, combined with strong winds, often causes flash floods, landslides, and widespread environmental damage.
6. Thermal Behavior of Water
(a) Role in Climate Control
Large water bodies store and release heat, stabilizing temperatures in nearby regions and reducing weather extremes.
(b) Effect of Density Variation
Water is most dense at 4°C, which explains thermal layering in lakes and supports aquatic ecosystems during colder months.
(c) Key Thermal Values
- Melting Point: 0°C
- Boiling Point: 100°C
- Specific Heat Capacity: 4.18 J/g°C
- Latent Heat of Fusion: 334 J/g
- Latent Heat of Vaporization: 2260 J/g
7. Life Under Frozen Water Bodies
Ice forms on the surface of ponds, but water beneath remains at 4°C, providing a survivable environment for aquatic animals during winter.
8. How Water’s Properties Differ from Hydrogen and Oxygen
Unlike its gaseous components, water is liquid at room temperature and exhibits unique behaviors like surface tension and polarity.
9. Survival Advantage of Water’s Density Anomaly
Because water’s highest density is at 4°C, aquatic life can thrive beneath frozen surfaces during winter months.
10. Simple Experiments Demonstrating Water’s Content
- Dissolved salts: Evaporate tap water to observe solid residues.
- Dissolved gases: Gentle heating shows bubble formation prior to boiling.
11. Importance of Gases Dissolved in Water
CO2 supports aquatic plant life through photosynthesis, while O2 is vital for fish and other underwater animals.
12. Difference Between Dissolved Air and Atmospheric Air
Water holds more dissolved oxygen compared to the air we breathe, which is essential for aquatic respiration.
13. Why Large Water Bodies Resist Freezing
Water’s ability to release latent heat delays freezing in lakes and rivers, ensuring that deeper layers remain liquid.
14. The Role of Minerals in Aquatic Environments
Dissolved minerals help maintain osmotic balance and provide key nutrients for aquatic organisms.
15. Further Explanations
- Flat taste of distilled water: It lacks minerals and gases.
- Ice cools better than water at 0°C: Because it absorbs latent heat while melting.
- Steam burns more severely: Due to additional latent heat of vaporization.
- Rainwater purity: Leaves no mineral stains when evaporated.
- Distilled water etching glass: Reactivity with glass over time creates etch marks.
16. Understanding Water’s Phase Changes
Water’s phase diagram explains the energy exchanges involved in melting, freezing, evaporation, and condensation processes.
🔗 Related Resource:
Explore Water Cycle Concepts for Grade 9
For more science tutorials and solved questions, visit more-marks.com.



